Die Hauptrolle der Lipidierung von Omega-3-Fettsäuren beim nicht-vegetativen Altern

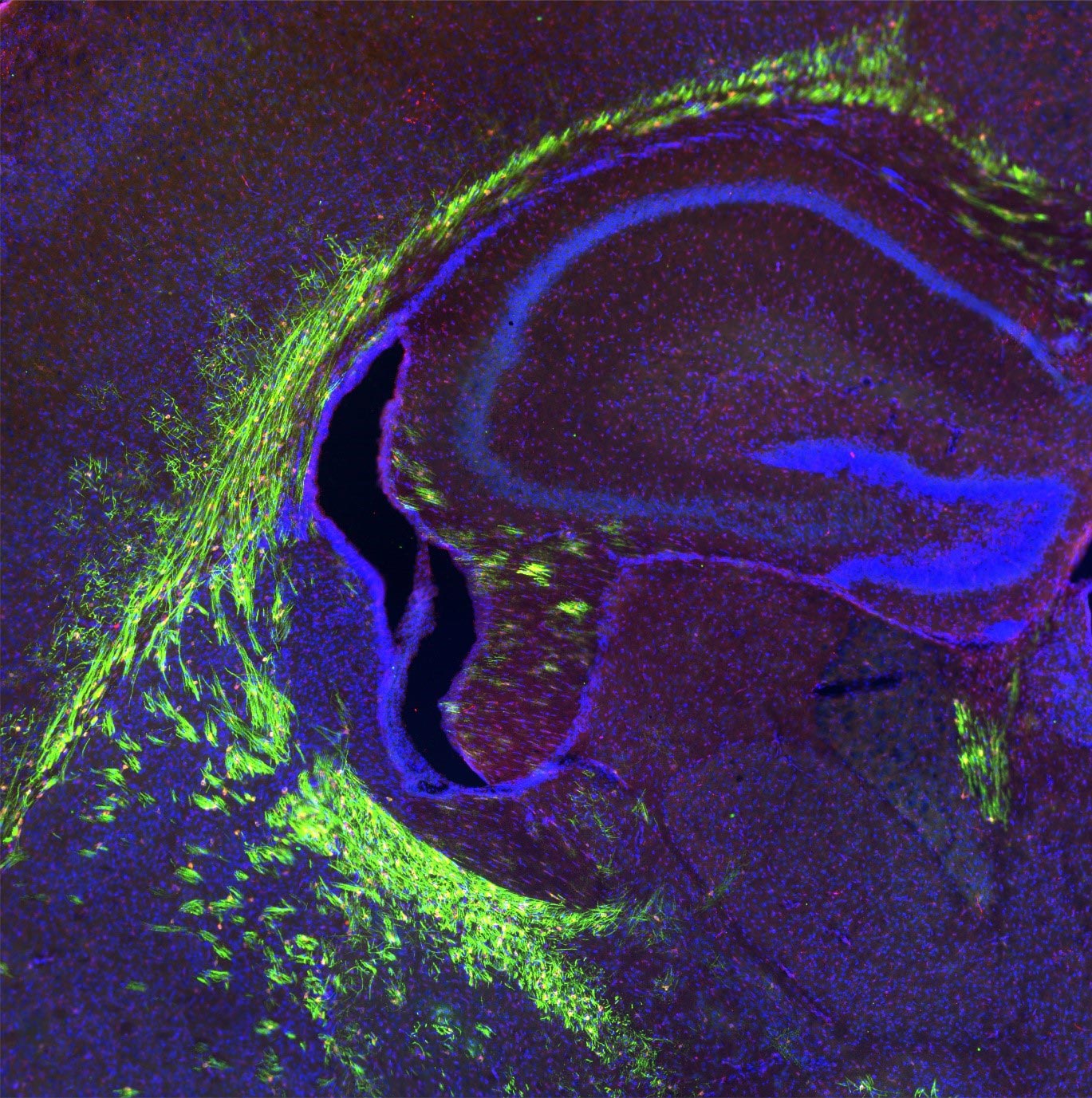
Gehirn des sich entwickelnden präklinischen Modells mit myelinisierten Axonen (in grün dargestellt). Wissenschaftler aus Singapur haben die entscheidende Rolle eines Transporterproteins, Mfsd2a, bei der Regulierung von Gehirnzellen entdeckt, die Myelinscheiden, die isolierende Membran, die Nerven umhüllt, aufrechterhalten. Diese Ergebnisse veröffentlicht in Zeitschrift für klinische UntersuchungEs kann helfen, die Auswirkungen des Alterns auf das Gehirn zu reduzieren. Mfsd2a transportiert Lysophosphatidylcholin (LPC), ein Lipid, das Omega-3-Fettsäuren enthält, zur Myelogenese zum Gehirn. Bildnachweis: Dr. Vetrivel Sengotuvil
Forscher haben entdeckt, dass das Mfsd2a-Transporterprotein für die Regulierung von Gehirnzellen unerlässlich ist, die die Myelinscheiden aufrechterhalten, die die Nerven schützen. Diese Entdeckung könnte dazu beitragen, die Auswirkungen des Alterns auf das Gehirn zu reduzieren und zu Behandlungen für neurologische Störungen führen, die durch eine reduzierte Myelogenese verursacht werden.
Wissenschaftler aus Singapur haben gezeigt, welche entscheidende Rolle ein spezielles Transportprotein bei der Regulierung von Gehirnzellen spielt, die dafür sorgen, dass Nerven durch Hüllen, sogenannte Myelinscheiden, geschützt werden. Die Ergebnisse, veröffentlicht von Forschern der Duke-NUS Medical School und der National University of Singapore in Zeitschrift für klinische UntersuchungEs kann helfen, die schädlichen Auswirkungen des Alterns auf das Gehirn zu reduzieren.
Als isolierende Membran, die Nerven umgibt, erleichtern Myelinscheiden die schnelle und effiziente Weiterleitung elektrischer Signale durch das gesamte Nervensystem des Körpers. Wenn die Myelinscheide beschädigt ist, können Nerven ihre Funktionsfähigkeit verlieren und neurologische Störungen verursachen. Mit zunehmendem Alter können die Myelinscheiden auf natürliche Weise beginnen, sich zu verschlechtern, weshalb ältere Menschen ihre körperlichen und geistigen Fähigkeiten verlieren.
Der Verlust von Myelinscheiden tritt während des normalen Alterungsprozesses und bei neurodegenerativen Erkrankungen wie Multipler Sklerose auf[{“ attribute=““>Alzheimer’s disease,” said Dr. Sengottuvel Vetrivel, Senior Research Fellow with Duke-NUS’ Cardiovascular & Metabolic Disorders (CVMD) Program and lead investigator of the study. “Developing therapies to improve myelination—the formation of the myelin sheath—in aging and disease is of great importance to ease any difficulties caused by declining myelination.”
To pave the way for developing such therapies, the researchers sought to understand the role of Mfsd2a, a protein that transports lysophosphatidylcholine (LPC)—a lipid that contains an omega-3 fatty acid—into the brain as part of the myelination process. From what is known, genetic defects in the Mfsd2a gene leads to significantly reduced myelination and a birth defect called microcephaly, which causes the baby’s head to be much smaller than it should be.

Dr. Sengottuvel Vetrivel (left) and Prof David Silver (right). Credit: Duke-NUS Medical School
In preclinical models, the team showed that removing Mfsd2a from precursor cells that mature into myelin-producing cells—known as oligodendrocytes—in the brain led to deficient myelination after birth. Further investigations, including single-cell RNA sequencing, demonstrated that Mfsd2a’s absence caused the pool of fatty acid molecules—particularly omega-3 fats—to be reduced in the precursor cells, preventing these cells from maturing into oligodendrocytes that produce myelin.
“Our study indicates that LPC omega-3 lipids act as factors within the brain to direct oligodendrocyte development, a process that is critical for brain myelination,” explained Professor David Silver, the senior author of the study and Deputy Director of the CVMD Program. “This opens up potential avenues to develop therapies and dietary supplements based on LPC omega-3 lipids that might help retain myelin in the aging brain—and possibly to treat patients with neurological disorders stemming from reduced myelination.”
Previously, Prof Silver and his lab discovered Mfsd2a and worked closely with other teams to determine the function of LPC lipids in the brain and other organs. The current research provides further insights into the importance of lipid transport for oligodendrocyte precursor cell development.
“We’re now aiming to conduct preclinical studies to determine if dietary LPC omega-3 can help to re-myelinate damaged axons in the brain,” added Prof Silver. “Our hope is that supplements containing these fats can help to maintain—or even improve—brain myelination and cognitive function during aging.”
“Prof Silver has been relentless in investigating the far-reaching role of Msdf2a ever since he discovered this important lipid transport protein, alluding to the many possible ways of treating not only the aging brain but also other organs in which the protein plays a role,” said Professor Patrick Casey, Senior-Vice Dean for Research. “It’s exciting to watch Prof Silver and his team shape our understanding of the roles that these specialized lipids play through their many discoveries.”
Reference: “Deficiency in the omega-3 lysolipid transporter Mfsd2a leads to aberrant oligodendrocyte lineage development and hypomyelination” by Vetrivel Sengottuvel, Monalisa Hota, Jeongah Oh, Dwight L. Galam, Bernice H. Wong, Markus R. Wenk, Sujoy Ghosh, Federico Torta and David L. Silver, 27 April 2023, The Journal of Clinical Investigation.
DOI: 10.1172/JCI164118

„Böser Kaffee-Nerd. Analyst. Unheilbarer Speckpraktiker. Totaler Twitter-Fan. Typischer Essensliebhaber.“
